2AHV9-UB2550
LifeSignals, Inc.UbiqVue 2A Wearable Biosensor using UbiqVue 2A Wireless Patient Monitoring System
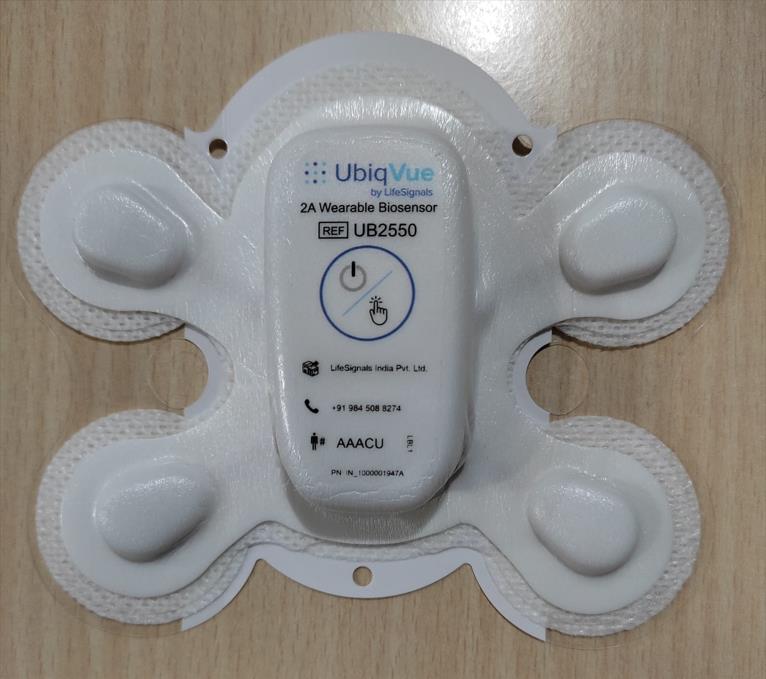
Click to zoom
Application Details
- Equipment Class
- DTS - Digital Transmission System
- Date of Grant
- May 28, 2023
- Application Purpose
- Original Equipment
- Date of Application
- May 28, 2023
- Equipment Note
- UbiqVue 2A Wearable Biosensor using UbiqVue 2A Wireless Patient Monitoring System
- Frequency Range
- 2412.00000000 - 2462.00000000
- Company
- LifeSignals, Inc.
- Country
- United States
Documents & Files
Select a file to view
Users Manual
Attestation Statements
Cover Letter(s)
External Photos
ID Label/Location Info
Internal Photos
RF Exposure Info
Test Report
Test Setup Photos
Contact Information
Applicant
Saravanan Balasubramanian(VP - Global Medical Technology & Regulatory Ops)
Technical Contact
LifeSignals, Inc.Saravanan Balasubramanian
[email protected]5107706412
Technical Specifications
| # | Rule Parts | Frequency Range | Power Output |
|---|---|---|---|
| 2 | 15C | 2.41 GHz - 2.46 GHz | 1.40 mW |